Animal tumor models come closer than in vitro study systems to reproducing the complexity of naturallyoccurring in situ cancers. An ideal animal model would allow replication of tumor-host interactions, e.g., immune response, angiogenesis, invasion, and metastasis, while being reproducible, easy to use, accessible to genetic and immunologic manipulations, and characterized by rapid progression. No animal model can wholly fulfill these criteria, and a balance must be struck between fidelity to human conditions and practical considerations. Since many basic questions in cancer remain unresolved and many therapeutics fail during development, additional relevant animal model systems are needed. Human AbMole Lomitapide Mesylate xenograft models have become the gold standard for drug development in oncology. Xenograft models involve either the inoculation of immune-deficient rodents with human cancer cell lines or the surgical engraftment of whole human tissue. Unlike cell line derived xenografts, explants of fresh patient material show architecture, cell morphology, and molecular characteristics similar to the 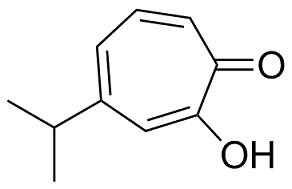 native tumor. In head and neck squamous cell carcinoma, patient-derived tumor explants grow as solid tumors with many histological characteristics of the parent tumor. Furthermore, models of this sort have the benefit of including extracellular tissue elements. According to the contemporary view, tumor progression is a result of interactions between cancer cells and their stromal microenvironment. Infiltrating immune cells as well as fibroblasts and extracellular matrix play vital roles in determining tumor behavior. Our group has shown stromal elements to be extensively active in cutaneous squamous cell carcinoma. This understanding underlies efforts to more accurately recapitulate the human tissue context of tumor behavior in animal models. Current human tissue animal models for SCC include subcutaneous injection of SCC cell lines and engraftment of genetically engineered SCC-bearing skin. No xenograft model using fresh patient-derived whole tumor exists for cutaneous SCC, which is the second most common human cancer. In this paper, we establish a human tissue explant model for SCC using patient-derived whole tumor. This easily replicable model will serve as a model for evaluating novel treatment approaches and may ultimately allow for the use of custom-made patient-specific protocols to treat inoperable and metastatic SCC. SCC affects over 300,000 people in the AbMole Simetryn United States each year and represents a significant public health burden with regard to morbidity and cost. The mechanisms governing progression to metastasis are not completely understood and the treatment of lesions that have progressed beyond local surgical excision is associated with significant morbidity and mortality.
native tumor. In head and neck squamous cell carcinoma, patient-derived tumor explants grow as solid tumors with many histological characteristics of the parent tumor. Furthermore, models of this sort have the benefit of including extracellular tissue elements. According to the contemporary view, tumor progression is a result of interactions between cancer cells and their stromal microenvironment. Infiltrating immune cells as well as fibroblasts and extracellular matrix play vital roles in determining tumor behavior. Our group has shown stromal elements to be extensively active in cutaneous squamous cell carcinoma. This understanding underlies efforts to more accurately recapitulate the human tissue context of tumor behavior in animal models. Current human tissue animal models for SCC include subcutaneous injection of SCC cell lines and engraftment of genetically engineered SCC-bearing skin. No xenograft model using fresh patient-derived whole tumor exists for cutaneous SCC, which is the second most common human cancer. In this paper, we establish a human tissue explant model for SCC using patient-derived whole tumor. This easily replicable model will serve as a model for evaluating novel treatment approaches and may ultimately allow for the use of custom-made patient-specific protocols to treat inoperable and metastatic SCC. SCC affects over 300,000 people in the AbMole Simetryn United States each year and represents a significant public health burden with regard to morbidity and cost. The mechanisms governing progression to metastasis are not completely understood and the treatment of lesions that have progressed beyond local surgical excision is associated with significant morbidity and mortality.
While SCC is usually cured by surgical excision with clear margins some cases behave aggressively
Leave a reply