We and others have found that this assay is 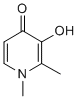 sensitive to low levels of oligomers that do not cause cell death. The dual applicability of this assay to measure both cell death and membrane trafficking rate under different conditions has led to some confusion in the literature, particularly since low amounts of oligomers that lead to inhibition of LTP do not lead to cell death. All of our assays are conducted with non-lethal concentrations of Abeta oligomers. There are large differences in the potency of synthetic Abeta oligomers and human derived Abeta in the trafficking assay, perhaps due to the presence of large amounts of monomer in synthetic preps. However, we have found that the ability of compounds to block the effects of multiple sources of Abeta oligomers without having effects on their own is predictive for their ability to restore MDL-29951 cognitive function in in vivo models of Alzheimer’s disease caused by age-related increases in Abeta. Thus, although our knowledge of the exact pathological species of Abeta oligomer is incomplete, by using multiple assays and multiple preps we have discovered diseasemodifying therapeutic candidates with the potential to modify the course of the disease. We have linked this membrane trafficking assay with Abeta binding and synapse counting assays into a platform for discovering anti-Abeta drugs which restore cognitive ability in vivo. The binding of synthetic Abeta oligomers to puncta on neuronal neurites shows concordance with their functional effect in altering trafficking rates, with a binding affinity agreeing with an EC50 in the functional assay. The concentration of compounds needed to inhibit binding of Abeta oligomers is in good agreement with the concentration which inhibits the effects of Abeta oligomers on membrane trafficking and synapse loss in vitro. For example, CT01344 blocked the effects of Abeta on membrane trafficking with an EC50 of 8.760.4 mM and displaced Abeta binding on cultured neurons with an EC50 of 3.961.8 mM. CT01344 restored synapse loss induced by Abeta 100% at 15 mM The low dynamic range of synapse loss seen with the non-lethal concentrations of synthetic oligomers used in all our experiments makes it difficult to observe a dose-dependent effect. The concentration of compound used is at the top of the dose-response curve for CT01344 inhibition of oligomer-induced membrane trafficking deficits. Thus, there is a good correlation between potency of the synthetic Abeta oligomer preparation in the different in vitro assays as well as a good correlation with potency of the compounds in those assays. In behavioral studies in transgenic mice, all four of the compounds we have studied must reach a brain concentration that exceeds a theoretical receptor occupancy at Folic acid sigma-2/ PGRMC1 of greater than 80% to be behaviorally effective. The EC50 for the effects of CT01344 in vitro is nearly two orders of magnitude above its binding affinity for sigma-2/ PGRMC1 as determined by radioligand binding studies. This offset between affinity at sigma-2/PGRMC1 could be due to one or more factors: 1) physico-chemical interactions of the compound such as binding to microtiter plate plastic, could lower the effective concentration of the compound in in vitro assays, 2) greater than 95% of sigma-2/PGRMC1 receptors need to be occupied by compound to achieve an effect on binding and function, or 3) the high concentration of the low potency synthetic Abeta needed to achieve adequate testing windows in the in vitro assays. The first possibility likely plays some role, due to the relative lipophilicity of these compounds �C a property which is desired for compounds to penetrate the bloodbrain barrier.
sensitive to low levels of oligomers that do not cause cell death. The dual applicability of this assay to measure both cell death and membrane trafficking rate under different conditions has led to some confusion in the literature, particularly since low amounts of oligomers that lead to inhibition of LTP do not lead to cell death. All of our assays are conducted with non-lethal concentrations of Abeta oligomers. There are large differences in the potency of synthetic Abeta oligomers and human derived Abeta in the trafficking assay, perhaps due to the presence of large amounts of monomer in synthetic preps. However, we have found that the ability of compounds to block the effects of multiple sources of Abeta oligomers without having effects on their own is predictive for their ability to restore MDL-29951 cognitive function in in vivo models of Alzheimer’s disease caused by age-related increases in Abeta. Thus, although our knowledge of the exact pathological species of Abeta oligomer is incomplete, by using multiple assays and multiple preps we have discovered diseasemodifying therapeutic candidates with the potential to modify the course of the disease. We have linked this membrane trafficking assay with Abeta binding and synapse counting assays into a platform for discovering anti-Abeta drugs which restore cognitive ability in vivo. The binding of synthetic Abeta oligomers to puncta on neuronal neurites shows concordance with their functional effect in altering trafficking rates, with a binding affinity agreeing with an EC50 in the functional assay. The concentration of compounds needed to inhibit binding of Abeta oligomers is in good agreement with the concentration which inhibits the effects of Abeta oligomers on membrane trafficking and synapse loss in vitro. For example, CT01344 blocked the effects of Abeta on membrane trafficking with an EC50 of 8.760.4 mM and displaced Abeta binding on cultured neurons with an EC50 of 3.961.8 mM. CT01344 restored synapse loss induced by Abeta 100% at 15 mM The low dynamic range of synapse loss seen with the non-lethal concentrations of synthetic oligomers used in all our experiments makes it difficult to observe a dose-dependent effect. The concentration of compound used is at the top of the dose-response curve for CT01344 inhibition of oligomer-induced membrane trafficking deficits. Thus, there is a good correlation between potency of the synthetic Abeta oligomer preparation in the different in vitro assays as well as a good correlation with potency of the compounds in those assays. In behavioral studies in transgenic mice, all four of the compounds we have studied must reach a brain concentration that exceeds a theoretical receptor occupancy at Folic acid sigma-2/ PGRMC1 of greater than 80% to be behaviorally effective. The EC50 for the effects of CT01344 in vitro is nearly two orders of magnitude above its binding affinity for sigma-2/ PGRMC1 as determined by radioligand binding studies. This offset between affinity at sigma-2/PGRMC1 could be due to one or more factors: 1) physico-chemical interactions of the compound such as binding to microtiter plate plastic, could lower the effective concentration of the compound in in vitro assays, 2) greater than 95% of sigma-2/PGRMC1 receptors need to be occupied by compound to achieve an effect on binding and function, or 3) the high concentration of the low potency synthetic Abeta needed to achieve adequate testing windows in the in vitro assays. The first possibility likely plays some role, due to the relative lipophilicity of these compounds �C a property which is desired for compounds to penetrate the bloodbrain barrier.
The binding site of the compounds is not identical to the binding site of Abeta oligomers
Leave a reply