Although the strong phenotypic effects we observe on gastrulation, neural tube closure, and Keller explants could reflect direct participation of Hipk1 in a b-catenin-independent pathway such as PCP, the most parsimonious model is that Hipk1 functions upstream of these processes in Wnt/b-catenin transcriptional activation during gastrulation. This is consistent with our timelapse analysis of Echinatin gastrulation movements in Hipk1 morphant embryos, which documents changes as early as Stage 10 when specification of the mesoderm occurs. In future studies it will be interesting to examine whether Hipk1 helps regulate transcription of PCP pathway component genes, and even whether it interacts with PCP proteins such as Dsh in a cytoplasmic compartment, beyond its nuclear role in regulating transcription. We have also observed in X. laevis embryos and in mammalian cells that Hipk1 can antagonize Wnt/b-catenin-mediated gene activation. Previous studies have characterized members of the Hipk protein family as transcriptional co-repressors. This, combined with the biochemical interaction of Hipk1 with Tcf3 and the broadened expression domains of dorsal patterning genes in Hipk1 morphants, suggests that Hipk1 and Tcf3 may cooperatively restrict activation of 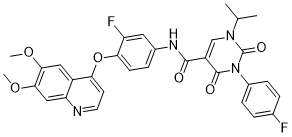 certain Wnt/b-catenin pathway targets to a circumscribed region of the dorsal embryo. It is interesting that effects of Hipk1 on the Wnt/b-catenin pathway depend on an intact kinase domain, and that this kinase activity also appears to negatively regulate the strength of this Hipk1/Tcf3 interaction. This suggests that cooperative functions of Hipk1 and Tcf3 may be regulated by the Hipk1 kinase. In this case Tcf3 or another factor in the same transcriptional complex such as Ginsenoside-F5 Groucho might be a Hipk1 kinase substrate. One implication is that the expression of some developmentally-important genes may be coordinated by pathways that regulate Hipk1 kinase activity in conjunction with Wnt/b-catenin signaling. It is less clear from our present study how Dsh may be mechanistically involved in the gene-regulatory functions of Hipk1. Although Dsh proteins are considered to be predominantly cytoplasmic, they also can localize to the nucleus and have been reported to interact with c-Jun and Tcf proteins. As Hipk1 is predominantly nuclear and as it regulates gene transcription, we speculate that it may interact with a nuclear complex containing.
certain Wnt/b-catenin pathway targets to a circumscribed region of the dorsal embryo. It is interesting that effects of Hipk1 on the Wnt/b-catenin pathway depend on an intact kinase domain, and that this kinase activity also appears to negatively regulate the strength of this Hipk1/Tcf3 interaction. This suggests that cooperative functions of Hipk1 and Tcf3 may be regulated by the Hipk1 kinase. In this case Tcf3 or another factor in the same transcriptional complex such as Ginsenoside-F5 Groucho might be a Hipk1 kinase substrate. One implication is that the expression of some developmentally-important genes may be coordinated by pathways that regulate Hipk1 kinase activity in conjunction with Wnt/b-catenin signaling. It is less clear from our present study how Dsh may be mechanistically involved in the gene-regulatory functions of Hipk1. Although Dsh proteins are considered to be predominantly cytoplasmic, they also can localize to the nucleus and have been reported to interact with c-Jun and Tcf proteins. As Hipk1 is predominantly nuclear and as it regulates gene transcription, we speculate that it may interact with a nuclear complex containing.
These other genes are not required for induction of Wnt/b-catenin target genes such as Xbra or otx2
Leave a reply