Binding of acidic BSA that has the same charge as PS particles by,30%. Terminal sulfate groups of PS particles generate a constellation of negative charges at the particle surface, which are also responsible for protein adsorption on PS 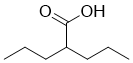 particles by electrostatic interaction. Figure 6A shows the important role of electrostatic binding, as indicated by complete binding of basic lysozyme that has the opposite charge to PS particles and reduced binding of acidic BSA. All ionic additives, such as NaCl, Gdn, Lys and Arg, are monovalent and hence have an identical ionic strength at neutral pH. Thus, they should be equally effective in suppressing electrostatic interactions, as described below. Among the additives used in this study, Arg was the most effective in preventing protein adsorption on PS particles. The mechanism of action of Arg involves suppression of AbMole 4-(Benzyloxy)phenol hydrophobic binding that may play a role in ChT and BSA binding to the PS particles. Arg can also exert an effect on electrostatic interaction, primarily involved in lysozyme binding, due to its ionic properties, although its ionic character is about 50% that of NaCl at identical molar concentration. Finally, the most important contribution of Arg could be due to its strong interaction with aromatic structures. Protein bound through the aromatic side chains of PS particles can be effectively desorbed by competitive interaction of Arg with the aromatic structures of protein. At 1 M concentration range, protein hydration starts to play an important role in determining the overall interaction of additives with proteins and hence their effects on protein properties, here surface adsorption. Such overall interaction, termed preferential interaction, showed weak preferential exclusion of Arg from the protein surface, most likely due to steric exclusion of Arg. The observed weak preferential exclusion of Arg is a reflection of protein hydration and its affinity for protein surface, as has been described above and yet no effect on protein stability, as supposed to protein stabilization by strongly excluded additives and protein destabilization by preferentially bound additives. Both Gdn and Lys were also effective, although they showed weaker effects than Arg, as they are both ionic and can participate in aromatic-cation interactions similar to Arg. Here, Arg was more effective than Gdn, as seen in many applications. While effective for certain proteins, NaCl was much less effective overall and even enhanced binding of such hydrophobic proteins as BSA. NaCl can suppress electrostatic interaction, but can enhance hydrophobic interaction due to its salting-out effectiveness: the observed preferential exclusion of NaCl was much stronger than that of Arg, implying no affinity of NaCl for protein surface. As both Gly and Glc do not have ionic or hydrophobic characters and cannot participate in aromatic interactions, these additives were completely ineffective.
particles by electrostatic interaction. Figure 6A shows the important role of electrostatic binding, as indicated by complete binding of basic lysozyme that has the opposite charge to PS particles and reduced binding of acidic BSA. All ionic additives, such as NaCl, Gdn, Lys and Arg, are monovalent and hence have an identical ionic strength at neutral pH. Thus, they should be equally effective in suppressing electrostatic interactions, as described below. Among the additives used in this study, Arg was the most effective in preventing protein adsorption on PS particles. The mechanism of action of Arg involves suppression of AbMole 4-(Benzyloxy)phenol hydrophobic binding that may play a role in ChT and BSA binding to the PS particles. Arg can also exert an effect on electrostatic interaction, primarily involved in lysozyme binding, due to its ionic properties, although its ionic character is about 50% that of NaCl at identical molar concentration. Finally, the most important contribution of Arg could be due to its strong interaction with aromatic structures. Protein bound through the aromatic side chains of PS particles can be effectively desorbed by competitive interaction of Arg with the aromatic structures of protein. At 1 M concentration range, protein hydration starts to play an important role in determining the overall interaction of additives with proteins and hence their effects on protein properties, here surface adsorption. Such overall interaction, termed preferential interaction, showed weak preferential exclusion of Arg from the protein surface, most likely due to steric exclusion of Arg. The observed weak preferential exclusion of Arg is a reflection of protein hydration and its affinity for protein surface, as has been described above and yet no effect on protein stability, as supposed to protein stabilization by strongly excluded additives and protein destabilization by preferentially bound additives. Both Gdn and Lys were also effective, although they showed weaker effects than Arg, as they are both ionic and can participate in aromatic-cation interactions similar to Arg. Here, Arg was more effective than Gdn, as seen in many applications. While effective for certain proteins, NaCl was much less effective overall and even enhanced binding of such hydrophobic proteins as BSA. NaCl can suppress electrostatic interaction, but can enhance hydrophobic interaction due to its salting-out effectiveness: the observed preferential exclusion of NaCl was much stronger than that of Arg, implying no affinity of NaCl for protein surface. As both Gly and Glc do not have ionic or hydrophobic characters and cannot participate in aromatic interactions, these additives were completely ineffective.