The present study was designed to investigate the in vitro effect of purpurin on C. dubliniensis biofilms, with special attention on its correlation to cell demise. The main etiopathologic role of biofilms in pathogenic Candida fungi is its ability to maintain a community of invasive fungal population that serves as a reservoir for recurrent host infection and disease dissemination. Similar to its phylogenetically closely related species C. albicans, C. dubliniensis forms true hyphae and biofilms on both abiotic and biotic surfaces. The increasing clinical prevalence of C. dubliniensis as an emerging human fungal pathogen is reflected by an astonishing number of reports in detecting bloodstream isolates and medical devices such as catheters and implanted materials. Our laboratory recently deciphered the mechanisms of action of purpurin against Candida fungi through perturbation of mitochondrial homeostasis and initiation of apoptosis. More experiments indicated that purpurin also interferes with C. albicans biofilm development at sub-MIC levels. In the present study, we explored the antifungal activity of purpurin on biofilms and evaluated several biochemical hallmarks of cell demise, an area that is still relatively unexplored in this Candida species. The antifungal activity of purpurin on biofilms was evaluated semi-quantitatively by using XTT Chamigrenal reduction assay. The inhibitory effect was concentration dependent, as revealed by a progressive reduction in cell viability with increasing concentrations of purpurin. This is an entirely new antifungal action mechanism which is different from the standard antifungal agents in clinical settings: flucytosine inhibits DNA synthesis; polyenes cause membrane damage; azoles affect sterol metabolism; and echinocandins inhibit cell wall synthesis. In conclusion, the findings of the present study provide solid evidence that purpurin triggered apoptosis-like features in C. dubliniensis biofilms. As C. dubliniensis and C. albicans belong to the CTG clade, it is not surprising that they share common core pathways in metabolism. Nonetheless, a comparative analysis of calcineurin signalling pathways between C. dubliniensis and C. albicans indicated differential scenarios in pH homeostasis. Another study revealed a rewiring of iron assimilation gene expression in C. dubliniensis. Thus, comparing metabolic pathways between these two species not only helps gain further insights into their evolutionary divergence, but also alludes to a functional characterization of core machineries responsible for their pathogenicity. For instance, a better understanding of the cell death mechanisms  can be beneficial to the design of innovative antifungal strategies that target the core components specifically. Anti-carbamyl-lysine antibodies have been in use for many years. We used a commercially available anti-carbamyl-lysine antibody to address whether carbamylated proteins are present in the kidneys of rats. As a preliminary confirmation of the specificity of the antibody, we incubated bovine serum albumin with urea for two to four days and immunoblotted the samples. After urea Isochlorogenic-acid-C incubation, the antibody recognized bovine serum albumin both as a monomer as well as in higher molecular weight complexes. In contrast, lanes loaded with samples prepared without urea did not show these bands. This result is compatible with the manufacturer’s data pointing to specificity of this antibody for carbamylated proteins. We used this antibody to probe immunoblots of rat renal cortex, outer medulla, and inner medulla. As hypothesized, many bands were seen in the inner medulla, consistent with the view that multiple carbamylated proteins are present in the inner medulla.
can be beneficial to the design of innovative antifungal strategies that target the core components specifically. Anti-carbamyl-lysine antibodies have been in use for many years. We used a commercially available anti-carbamyl-lysine antibody to address whether carbamylated proteins are present in the kidneys of rats. As a preliminary confirmation of the specificity of the antibody, we incubated bovine serum albumin with urea for two to four days and immunoblotted the samples. After urea Isochlorogenic-acid-C incubation, the antibody recognized bovine serum albumin both as a monomer as well as in higher molecular weight complexes. In contrast, lanes loaded with samples prepared without urea did not show these bands. This result is compatible with the manufacturer’s data pointing to specificity of this antibody for carbamylated proteins. We used this antibody to probe immunoblots of rat renal cortex, outer medulla, and inner medulla. As hypothesized, many bands were seen in the inner medulla, consistent with the view that multiple carbamylated proteins are present in the inner medulla.
Monthly Archives: April 2019
Carbamyl-cysteines located in internal portions of the proteins the primary antibody was omitted
Interestingly, we also observed extensive protein carbamylation in cortex and outer medulla, regions that have much lower physiological levels of urea. This finding is consistent with prior observations that proteins expressed in other organs with low local urea concentrations such as aquaporin, aBcrystallin, and TIMP-2 can undergo physiological carbamylation in the absence of elevated urea concentrations. Various proteins have also been shown to undergo increases in carbamylation in association with uremic levels of circulating urea. The mass spectrometry analysis of rat inner Kaempferide medulla identified 456 unique carbamylation sites in 403 proteins. Figure 4 shows the distribution of these carbamylation sites by their locations in the proteins. The largest group consists of sites that occur at the N-terminal amino acid of the proteins. In addition, a significant number of peptides showed carbamylation of N-termini that are nominally in internal regions of the protein. It is unlikely that these sites are established at the time of trypsinization of the samples because of the exhaustive procedures used to deplete the samples of urea. Indeed, 56 of 127 of these N-terminal sites were carbamylated at nontrypsin sites, i.e., at sites not preceded by lysine or arginine. We propose that these internal sites are present in the intact animal as a result of the action of physiological Gentiopicrin proteolytic processes, including the protein degradation that occurs as part of normal turnover of proteins in the cell. Note that many proteases in addition to trypsin, e.g. furin, cut after basic amino acids. Therefore, the remaining 71 sites may be targeted by endogenous members of this trypsin-like protease family. The results are summarized in Figure 6. Overall, slightly more than seven percent of identified proteins were found to be carbamylated and all compartments are represented. We speculate that the lower fraction of lysosomal proteins that are carbamylated may be because of the low pH inside  of lysosomes, which would titrate the cyanate ion to cyanic acid. This could alter the protein folding or its ability to interact with other macromolecules. Another possibility is that carbamylation of lysines, arginines, cysteines, and protein N-termini may prevent other post-translational modifications. For example, lysines undergo multiple post-translational modifications including sumoylation, ubiquitylation, and acetylation. These can have important consequences with regard to the function and stability of the target protein. For example, ubiquitylation is known to mark proteins for degradation either by the proteasome pathway or via lysosomes. In general the molecular weight inferred from the mass spectrometry studies agrees with the nominal molecular weight of the individual proteins. We conclude from these observations that protein carbamylation occurs endogenously in the kidney and is present in multiple proteins that play important physiological roles. However, contrary to the hypothesis expressed in the Introduction, protein carbamylation does not appear to be restricted to the inner medulla where urea is highest in the kidney. Nor is there a striking difference in carbamylation in the inner medulla as a result of variation in water intake, which is associated with striking changes in levels of tissue urea in the renal inner medulla. It seems likely that circulating levels of urea, typically 4 mM in nondiuretic rats, is sufficient to result in carbamylation, providing an explanation for previous findings that several nonrenal proteins can be carbamylated. Currently approximately 1,400 species placed into 29 formal subgenera and 39 informal species groups are recognized worldwide.
of lysosomes, which would titrate the cyanate ion to cyanic acid. This could alter the protein folding or its ability to interact with other macromolecules. Another possibility is that carbamylation of lysines, arginines, cysteines, and protein N-termini may prevent other post-translational modifications. For example, lysines undergo multiple post-translational modifications including sumoylation, ubiquitylation, and acetylation. These can have important consequences with regard to the function and stability of the target protein. For example, ubiquitylation is known to mark proteins for degradation either by the proteasome pathway or via lysosomes. In general the molecular weight inferred from the mass spectrometry studies agrees with the nominal molecular weight of the individual proteins. We conclude from these observations that protein carbamylation occurs endogenously in the kidney and is present in multiple proteins that play important physiological roles. However, contrary to the hypothesis expressed in the Introduction, protein carbamylation does not appear to be restricted to the inner medulla where urea is highest in the kidney. Nor is there a striking difference in carbamylation in the inner medulla as a result of variation in water intake, which is associated with striking changes in levels of tissue urea in the renal inner medulla. It seems likely that circulating levels of urea, typically 4 mM in nondiuretic rats, is sufficient to result in carbamylation, providing an explanation for previous findings that several nonrenal proteins can be carbamylated. Currently approximately 1,400 species placed into 29 formal subgenera and 39 informal species groups are recognized worldwide.
This is most likely due to the characteristics of one of the hybridization proble
Using primary antibodies against epithelial markers, disseminated tumor cells and CTC have been detected in bone marrow and blood from patients with various malignancies. Among a large variety of alternative strategies applied for CTCenrichment and analysis, an automated system for immunocytometric detection and quantitation of CTC has been developed, which was validated in breast, prostate, colorectal and lung 14alpha-hydroxy-Sprengerinin-C cancer patients. Interestingly, the absolute amount of CTC detected in advanced stage cancer patients varied considerably, depending on CTC-isolation techniques. While high numbers of CTC were detected in patients with small-cell lung cancer, absolute CTC numbers and dynamic range in NSCLC seemed too low for broad clinical applicability. Nevertheless, it has become increasingly clear that CTC populations are phenotypically heterogenous and epithelial-marker independent enrichment approaches are warranted to display the full picture of CTC in a cancer Forsythin patient at a given time point. Several recent reports supported this notion by consistently showing higher CTC numbers when comparing epithelial-marker-independent CTC enrichment techniques with the widely applied CTC isolation by selection of EpCAM and CKpositive cells using the CellSearch system. Against this background we reasoned whether probing for cancer-specific somatic mutations instead of microscopy-based parameters enhances the sensitivity of CTC detection in NSCLC. We have chosen EGFR DelEx19 mutations as a model to develop a highly sensitive, specific and robust method for mutation detection in mixtures of genomic DNA that were extracted from CTC-enriched peripheral blood cell populations. The novel method applied in this study has been designed to detect the vast majority of EGFR DelEx19 mutations, notably all possible mutant variants disrupting codons 746 and 747 of the EGFR gene. In contrast to a prior study analyzing only EpCAM-positive blood cells we have chosen an unbiased, epithelial markerindependent CTC enrichment strategy where samples were split and CTC enrichment was achieved by  a dual approach, depleting leucocytes in one half of a sample and selection of EpCAMpositive cells in the other half. This takes into account the phenotypic variability of CTC, which may lose one or several epithelial markers while being shed from a primary tumor or metastases. In pilot experiments, Immunofluorescence staining of selected CTC samples from NSCLC patients indeed showed heterogeneous populations of CTCs displaying epithelial, mesenchymal and mixed phenotypes. Applying this unbiased CTC-enrichment approach, we achieved a 100% detection rate in a pilot cohort of 8 patients with EGFR-mutant NSCLC. Most interestingly, we observed an association of EGFRmutant CTC with treatment response and outcome. These findings are most likely to be attributed to the novel and highly sensitive mutation detection method applied in this study. It has been recognized for some time that detection of mutations at levels below 1% is not reliably possible by next generation sequencing applications due to intrinsic error rates of this technology. Only very recently improved technologies were reported, rendering detection of mutations at levels of 0,1% and below possible. In keeping with these results, we were not able to detect EGFR Del19 mutations by next generation sequencing in genomic DNA of samples displaying unambiguous mutation signals in our newly developed mutation detection assay. These findings indicate that our newly developed mutation detection assay apparently displays higher sensitivity than standard next generation sequencing techniques. Another remarkable aspect of our mutation detection assay was the observation that different EGFR DelEx19 mutations gave rise to different melting curves.
a dual approach, depleting leucocytes in one half of a sample and selection of EpCAMpositive cells in the other half. This takes into account the phenotypic variability of CTC, which may lose one or several epithelial markers while being shed from a primary tumor or metastases. In pilot experiments, Immunofluorescence staining of selected CTC samples from NSCLC patients indeed showed heterogeneous populations of CTCs displaying epithelial, mesenchymal and mixed phenotypes. Applying this unbiased CTC-enrichment approach, we achieved a 100% detection rate in a pilot cohort of 8 patients with EGFR-mutant NSCLC. Most interestingly, we observed an association of EGFRmutant CTC with treatment response and outcome. These findings are most likely to be attributed to the novel and highly sensitive mutation detection method applied in this study. It has been recognized for some time that detection of mutations at levels below 1% is not reliably possible by next generation sequencing applications due to intrinsic error rates of this technology. Only very recently improved technologies were reported, rendering detection of mutations at levels of 0,1% and below possible. In keeping with these results, we were not able to detect EGFR Del19 mutations by next generation sequencing in genomic DNA of samples displaying unambiguous mutation signals in our newly developed mutation detection assay. These findings indicate that our newly developed mutation detection assay apparently displays higher sensitivity than standard next generation sequencing techniques. Another remarkable aspect of our mutation detection assay was the observation that different EGFR DelEx19 mutations gave rise to different melting curves.
Underlie cochaperone-specific modulation of the ATPase activity
Collectively, structural and functional studies have suggested a mechanistic model of the NLR recruitment and maturation by the Hsp90Sgt1-Rar1 complex. In this model, ATP binding to the Hsp90-NTD of apo-Hsp90 in the open form can induce a fast dynamic exchange between a nucleotide-free Hsp90 and an ATP-bound state in which the lid segments and the Hsp90NTDs are in the open position. In the proposed mechanistic AbMole Tulathromycin B picture Rar1 is the key component of the regulatory assembly that can intersect the normal progression of the AbMole Povidone iodine ATPase cycle at the early stage of the cycle and inhibit the formation of the closed Hsp90 dimer, while accelerating the ATPase activity. Hence, cochaperone-mediated arrest of the Hsp90-ATPase conformational cycle can promote the assembly of the ternary Hsp90-Sgt1-Rar1 complex and recruitment of the NLR clients. Although it is established that the formation of the regulatory Hsp90 complexes with Sgt1 and Rar1 enable targeted modulation of the ATPase conformational cycle, molecular and energetic determinants of allosteric regulation have remained frustratingly elusive. Among important questions that are currently under active investigation are how do Rar1 and Sgt1 cooperate at the molecular level to mechanistically regulate Hsp90? how can Rar1 enhance the ATPase activity of Hsp90? is there a feasible unified mechanism that can explain the maturation process of NLR proteins by the Hsp90-Sgt1-Rar1 complex? Molecular understanding of the regulatory mechanisms critically depends on high-resolution structures of recognition-competent client states in regulatory complexes with the Hsp90-Sgt1Rar1 chaperone system. However, the dynamic nature of these molecular assemblies hinders the molecular details. Compounded by marginal stability of the regulatory complexes, structural and thermodynamic characterizations of the Hsp90 interactions remain technically challenging, which is evident from a relatively small number of high-resolution structures of the Hsp90-cochaperone complexes. Consequently, computational modeling of transient Hsp90-cochaperone interactions may complement structure-functional studies and provide molecular insights into mechanistic aspects of allosteric regulation of Hsp90. The transient nature and cooperativity of the Hsp90-cochaperone interactions necessitates a multi-scale modeling strategy that combines all-atom and coarse-grained representations of the biological system. The key to understanding dynamics and stability of the regulatory complexes is to provide a quantitative characterization of the dynamics and stability of the Hsp90cochaperone complexes; and to establish a linkage between cochaperone-induced global conformational changes in Hsp90 and specific interaction networks that can inhibit or promote progression of the ATPase cycle and thus control the recruitment of client proteins. Although principal modes of protein motions can be extracted from all-atom molecular dynamics simulations, coarsegrained approaches and elastic network models such as Gaussian network model combined with the normal mode analysis can efficiently probe functional movements by reducing protein structure representation to a network of uniformly connected nodes where the native interactions in the equilibrium structure determine conformational dynamics of the system. Computational studies have employed dynamic approaches to model collective motions and allosteric interactions in the Hsp90 crystal structures revealing conserved functional motifs that act collectively as central regulators of the chaperone dynamics and activity. Allatom simulations of the Hsp90  crystal structures from different species have detected two inter-domain hinge sites regulating allosteric interactions of the chaperone.
crystal structures from different species have detected two inter-domain hinge sites regulating allosteric interactions of the chaperone.
The introduction of the Hib UII failed to induce angiogenesis in NOX2 knockdown
In NOX2 knockout mice, and that UII increased NOX2 transcription in endothelial cells. Moreover, it has been shown that UII significantly increases ROS levels in pulmonary artery smooth muscle cells, and this response was accompanied by elevated protein levels of the NADPH oxidases subunits p22-phox 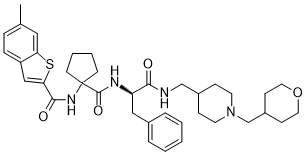 and NOX4. In addition, Chen et al showed that UII increased ROS production in cardiac fibroblasts. Our results demonstrated that UII significantly increased ROS levels in C2C12 cells, and this effect was accompanied by elevated protein translocation of the NADPH oxidase subunits p47-phox and p67-phox. These findings demonstrate for the first time that UII increases ROS production and NADPH oxidases subunits translocations in C2C12 cells. Glucose transport is mediated by insulin receptor substrate-1, phosphatidylinositol 3-kinase, and other signaling molecules, and down stream substrates activated by PI-3-kinase include protein kinase B /Akt and protein kinase C-��. Treatment of C2C12 cells with UII significantly decreased levels of GLUT4 translocation and phospho-AKT activated by insulin, and apocynin reversed these effects. We also AbMole Terbuthylazine determined that UII decreased phosphorylation of ERK1/2 and p38 MAPK, which are known mediators of glucose transport, and that apocynin improved these effects. However, treatment of C2C12 cells with UII had no effect on phospho-AKT, ERK and p38 MAPK levels, maybe because C2C12 cell is different from vascular smooth muscle cell. Collectively, these results demonstrated that UII impaired AbMole Riociguat BAY 63-2521 skeletal muscle glucose transport signaling pathways partly via a mechanism involving ROS production. In this study, we found some effects of urantide in vivo are different from those of UII in vitro. For example, p-PKC was increased by urantide in vivo but it was not changed by UII in vitro, however, p38MAPK was not changed in vivo, whereas it was decreased by UII. A close relationship between chronic inflammation and skeletal muscle insulin resistance had been established, so the reasons for these differences may be due to the role of inflammatory cytokine. Thus it should further discuss if these proteins are also phosphorylated by inflammatory cytokines. Pneumonia is the main cause of death in children worldwide. It is estimated that it kills 1��2 million children under five years every year, accounting for 18% of all deaths in this population group. Streptococcus pneumoniae and Haemophilus influenzae b are the two principal causes of bacterial pneumonia, and also major causes of other invasive bacterial diseases. These pathogens can be prevented by immunization or treated with low cost antibiotics but it has been estimated that only 30% of children with bacterial pneumonia receive the antibiotics that they need.
and NOX4. In addition, Chen et al showed that UII increased ROS production in cardiac fibroblasts. Our results demonstrated that UII significantly increased ROS levels in C2C12 cells, and this effect was accompanied by elevated protein translocation of the NADPH oxidase subunits p47-phox and p67-phox. These findings demonstrate for the first time that UII increases ROS production and NADPH oxidases subunits translocations in C2C12 cells. Glucose transport is mediated by insulin receptor substrate-1, phosphatidylinositol 3-kinase, and other signaling molecules, and down stream substrates activated by PI-3-kinase include protein kinase B /Akt and protein kinase C-��. Treatment of C2C12 cells with UII significantly decreased levels of GLUT4 translocation and phospho-AKT activated by insulin, and apocynin reversed these effects. We also AbMole Terbuthylazine determined that UII decreased phosphorylation of ERK1/2 and p38 MAPK, which are known mediators of glucose transport, and that apocynin improved these effects. However, treatment of C2C12 cells with UII had no effect on phospho-AKT, ERK and p38 MAPK levels, maybe because C2C12 cell is different from vascular smooth muscle cell. Collectively, these results demonstrated that UII impaired AbMole Riociguat BAY 63-2521 skeletal muscle glucose transport signaling pathways partly via a mechanism involving ROS production. In this study, we found some effects of urantide in vivo are different from those of UII in vitro. For example, p-PKC was increased by urantide in vivo but it was not changed by UII in vitro, however, p38MAPK was not changed in vivo, whereas it was decreased by UII. A close relationship between chronic inflammation and skeletal muscle insulin resistance had been established, so the reasons for these differences may be due to the role of inflammatory cytokine. Thus it should further discuss if these proteins are also phosphorylated by inflammatory cytokines. Pneumonia is the main cause of death in children worldwide. It is estimated that it kills 1��2 million children under five years every year, accounting for 18% of all deaths in this population group. Streptococcus pneumoniae and Haemophilus influenzae b are the two principal causes of bacterial pneumonia, and also major causes of other invasive bacterial diseases. These pathogens can be prevented by immunization or treated with low cost antibiotics but it has been estimated that only 30% of children with bacterial pneumonia receive the antibiotics that they need.