Effects on HUVECs to the natural-source compound 1. Endothelial cells from different tissues have different gene expression patterns suggesting different physiological effects. HUVECs are macrovascular endothelial cells which do not have specific relevance to the microvascular endothelial cells that are present in retinal capillaries of the eye. Therefore, we tested SH11052 in HRECs, where it proved similarly potent as an antiproliferative molecule, albeit at higher GI50, consistent with the hypothesis that microvascular endothelial cells differ from macrovascular endothelial cells. SH-11052 blocks proliferative progression through DNA synthesis in both HUVECs and HRECs. This is consistent with the documented G2/M phase cell cycle arrest induced by 1 in HUVECs. We have also demonstrated the in vitro anti-angiogenic activity of 2 in a Matrigel HREC tube formation assay, similar to the effects of 1 in HUVECs. The novel anti-angiogenic mechanism of homoisoflavanones remains largely unexplored. In HUVECs, compound 1 induced expression of p21WAF1, an inhibitor of the cyclindependent kinase Cdc2, which in turn is BIBW2992 downregulated by compound 1. Homoisoflavanone 1 also blocked prostaglandin synthesis from arachidonic acid in a microsome assay, without marked effects on WZ4002 function of cyclooxygenases 1 and 2 as purified enzymes. In keratinocytes, compound 1 inhibited the nuclear translocation of NF-kB under ultraviolet light-induced inflammatory conditions, suggesting a role of the compound in modulating inflammatory signals in these cells. In this context, compound 1 also decreased phosphorylation of the MAPKs Jun N-terminal kinase, p38 MAPK, and ERK. We examined if the activity of SH-11052 in HRECs may likewise be mediated through modulation of inflammatory signals. As NF-kB is the principal mediator of inflammation induced signals, we monitored NF-kB activation upon TNF-a stimulation in the absence or presence of compound 2 in HRECs. NF-kB is a transcription factor sequestered in the cytoplasm by association with IkB-a protein. Upon TNF-a stimulation, IkB-a is phosphorylated and degraded by the proteasome, releasing free NF-kB. The free NF-kB is then translocated into the nucleus and aids in the transcription of its target genes. Hence monitoring the protein levels of IkB-a and nuclear translocation of NF-kB upon TNF-a treatment are measures of the activation of the NF-kB pathway by inflammatory signals. Indeed, we show that IkBa degradation and nuclear translocation of NF-kB in HRECs are inhibited by compound 2. Furthermore, compound 2 also inhibited the expression of NF-kB inducible pro-angiogenic and pro-inflammatory genes, suggesting a role for compound 2 in the inhibition of inflammation-induced pro-angiogenic signaling in HRECs. SH-11052’s suppressive effects on expression of IL8 and PTGS2 in HRECs are consistent with the observed effects of the natural product 1 in keratinocytes. To our knowledge, we show for the first time an effect of a homoisoflavanone on the endothelial activation marker and NF-kB target, VCAM-1, and 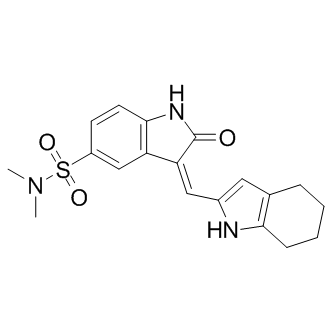 on the inflammatory marker CCL2. Thus, the data presented here are consistent with a function for compound 2 as an inhibitor of NFkB signaling in HRECs. NF-kB has previously been implicated in pathological ocular angiogenesis and we further confirmed that two known NF-kB pathway inhibitors, BAY 11-7082 and CAPE, had antiproliferative effects on HRECs. This assertion of an NF-kB-dependent role for compound 2 can integrate others’ findings regarding the activities of the related compound 1 in other cell types as well. bFGF can act by signaling through phospholipase Cc1, which activates protein kinase C a via diacylglycerol. In turn, PKCa binds and activates IKKa, which phosphorylates and inactivates I-kB.
on the inflammatory marker CCL2. Thus, the data presented here are consistent with a function for compound 2 as an inhibitor of NFkB signaling in HRECs. NF-kB has previously been implicated in pathological ocular angiogenesis and we further confirmed that two known NF-kB pathway inhibitors, BAY 11-7082 and CAPE, had antiproliferative effects on HRECs. This assertion of an NF-kB-dependent role for compound 2 can integrate others’ findings regarding the activities of the related compound 1 in other cell types as well. bFGF can act by signaling through phospholipase Cc1, which activates protein kinase C a via diacylglycerol. In turn, PKCa binds and activates IKKa, which phosphorylates and inactivates I-kB.
Monthly Archives: July 2019
It has been known that overexpression of members of the Bcl-2 rupture of outer mitochondrial membrane and bind with LRPPRC
Then, LRPPRC, Parkin and other substrates of Parkin may be ubiquitinated by Parkin E3 ligase and recognized by autophagy machinery and guide mitochondria to be degraded through mitophagy. Parkin is selectively recruited to dysfunctional mitochondria with low membrane potential in mammalian cells. After recruitment, Parkin mediates the engulfment of mitochondria by autophagosomes and the selective elimination of impaired mitochondria. Mitofusin 1, Drp1 and VDAC1 were reported to be substrates of Parkin while LRPPRC is also listed as Parkin substrates in the MG132  associated online supplementary although the exact mechanism is still in investigation. Ubiquitinated VDAC1 and Drp1 will cause their associated mitochondria to be brought into autophagosomes and autolysosomes for degradation. Since a significant portion of Bcl-2 is associated with mitochondria and Parkin-mono-ubiquitinated Bcl2 is more stable, suppression of LRPPRC leads to decreases in levels of Parkin and Bcl-2 and activation of basal autophagy as we previous reported. Interestingly, Parkin itself is the substrate of its ligase activity. After auto-ubiquitination, Parkin gradually becomes depleted along Bcl-2 and ATG5-ATG12 conjugate in cells under long-term mitophagy stress. The drug-induced mitophagy stress is an artificially introduced pathological condition. Under normal physiological condition, it is unlikely that all of mitochondria in cells are simultaneously damaged. The drug-induced mitochondrial damages are so massive that autophagy/mitophagy machinery is incapable of handing so many damaged mitochondria immediately. This is possibly the reason that we observed a large Nilotinib amount of mitochondria aggregates accumulated in the first 12 hrs after exposure to mitophagy inducer. These mitochondrial aggregates then become fragmented mitochondria to be engulfed in autophagosomes and further autolysosomes for degradation. Parkinson’s disease results from the death of dopaminecontaining cells in the substantia nigra region of the midbrain. Several mutations in specific genes such as Parkin have been identified in a few individuals with familial form or autosomal recessive juvenile Parkinson’s disease. Mitochondrial dysfunction and oxidative stress have long been implicated as the general pathophysiologic mechanisms underlying Parkinson’s disease. Impairment of autophagy and mitophagy processes may be the determining force in the majority of patients to develop Parkinson’s disease. Interestingly, the same group of proteins involved in juvenile Parkinson’s disease also plays important roles in tumorigenesis although the somatic mutations of Parkin identified are homozygous in Parkinson’s disease and heterozygous in cancers. If the autophagic process is blocked before autophagosomal formation, the fragmented mitochondria will release cytochrome c and other molecules to induce apoptosis that is usually associated with diverse forms of aggregation and perinuclear clustering of the dysfunctional mitochondria. If either the process is blocked before the autolysosomal formation or autophagosomes are not degraded efficiently, the accumulated mitochondria may become damaged by their own production of superoxide and start to leak electrons and lose their membrane potentials, and even further induce robust oxidative stress. High levels of oxidative stress are lethal in post-mitotic neuronal cells in Parkinson’s disease, while sub-lethal levels of oxidative stress not only induces DNA double-strand breaks but also weakens mitotic checkpoint function so that cells carrying damaged genomes can escape mitotic checkpoint to enter next rounds of mitosis to further destabilize the genomes and result in tumorigenesis. High levels of LRPPRC maintain Bcl-2 levels, block mitophagy and prevent mitochondria from autophagy degradation.
associated online supplementary although the exact mechanism is still in investigation. Ubiquitinated VDAC1 and Drp1 will cause their associated mitochondria to be brought into autophagosomes and autolysosomes for degradation. Since a significant portion of Bcl-2 is associated with mitochondria and Parkin-mono-ubiquitinated Bcl2 is more stable, suppression of LRPPRC leads to decreases in levels of Parkin and Bcl-2 and activation of basal autophagy as we previous reported. Interestingly, Parkin itself is the substrate of its ligase activity. After auto-ubiquitination, Parkin gradually becomes depleted along Bcl-2 and ATG5-ATG12 conjugate in cells under long-term mitophagy stress. The drug-induced mitophagy stress is an artificially introduced pathological condition. Under normal physiological condition, it is unlikely that all of mitochondria in cells are simultaneously damaged. The drug-induced mitochondrial damages are so massive that autophagy/mitophagy machinery is incapable of handing so many damaged mitochondria immediately. This is possibly the reason that we observed a large Nilotinib amount of mitochondria aggregates accumulated in the first 12 hrs after exposure to mitophagy inducer. These mitochondrial aggregates then become fragmented mitochondria to be engulfed in autophagosomes and further autolysosomes for degradation. Parkinson’s disease results from the death of dopaminecontaining cells in the substantia nigra region of the midbrain. Several mutations in specific genes such as Parkin have been identified in a few individuals with familial form or autosomal recessive juvenile Parkinson’s disease. Mitochondrial dysfunction and oxidative stress have long been implicated as the general pathophysiologic mechanisms underlying Parkinson’s disease. Impairment of autophagy and mitophagy processes may be the determining force in the majority of patients to develop Parkinson’s disease. Interestingly, the same group of proteins involved in juvenile Parkinson’s disease also plays important roles in tumorigenesis although the somatic mutations of Parkin identified are homozygous in Parkinson’s disease and heterozygous in cancers. If the autophagic process is blocked before autophagosomal formation, the fragmented mitochondria will release cytochrome c and other molecules to induce apoptosis that is usually associated with diverse forms of aggregation and perinuclear clustering of the dysfunctional mitochondria. If either the process is blocked before the autolysosomal formation or autophagosomes are not degraded efficiently, the accumulated mitochondria may become damaged by their own production of superoxide and start to leak electrons and lose their membrane potentials, and even further induce robust oxidative stress. High levels of oxidative stress are lethal in post-mitotic neuronal cells in Parkinson’s disease, while sub-lethal levels of oxidative stress not only induces DNA double-strand breaks but also weakens mitotic checkpoint function so that cells carrying damaged genomes can escape mitotic checkpoint to enter next rounds of mitosis to further destabilize the genomes and result in tumorigenesis. High levels of LRPPRC maintain Bcl-2 levels, block mitophagy and prevent mitochondria from autophagy degradation.
Simple supportive care was sufficient to ensure a good outcome for all patients in this series
We demonstrated that imidacloprid self-poisoning resulted in mostly minor toxicity with a case-fatality of 0%. This is favourable compared to outcomes with other insecticides, in particular the widely used organophosphorus compounds which commonly have a case fatality between 5 and 30%. The most severely poisoned patients were both administered antidotes used for the treatment of organophosphorus pesticides and this may have increased the apparent toxicity. Many patients may have had a moderate metabolic acidosis on admission. Tachycardia and hypertension have usually been reported in previous cases, and recurrent ventricular fibrillation was the reported cause of death in a 69 year-old woman with coronary artery disease. Only 2 patients in our case series developed any cardiovascular toxicity which was predominantly hypotension and biomarkers of cardiac toxicity were not elevated. While electrocardiographic monitoring was not conducted in these patients, blood pressure improved with intravenous fluids. Therefore serious arrhythmias were unlikely to have caused the hypotension. Biochemical abnormalities and rhabdomyolysis have been reported as potentially serious complications that might lead to mortality. Most of the patients in our series had normal CK and biochemistry with the exception of low venous bicarbonate. The cause of this is not clear given the other biochemical results, although diarrhoea may be contributory. It may also be due to acidic metabolites of imidacloprid such as 6chloronicotinic acid and other metabolites; however,Imidazole metabolic pathways of imidacloprid have not been extensively studied in humans. Direct mitochondrial toxicity from a component of the formulation may cause anaerobic metabolism and produce a lactic acidosis which may cause a moderate decrease in bicarbonate. In animals, imidacloprid penetrates the blood-brain barrier to only a very limited extent. While a decreased level of consciousness was uncommon in our study, prolonged sedation and respiratory depression was noted in two patients which may have been due to co-ingestion of ethanol. Transient respiratory impairment appeared to contribute to deaths reported in patients with severe poisoning where co-ingestion of ethanol was not reported. There are no specific antidotes for neonicotinoid poisoning in mammals. On the basis of our experience, symptomatic and supportive care is all that is required for the management of patients with acute imidacloprid poisoning. Treatment with oximes such as pralidoxime is expected to be either ineffective or contraindicated. Oximes in the absence of organophosphorus pesticides have a weak inhibitory effect on acetylcholinesterase activity and therefore might increase nicotinic effects. It is notable that our two most seriously poisoned cases received treatment with pralidoxime. The concentration-time profile shown in Figure 3 suggests that there is rapid absorption, with high concentrations being noted on admission. In rats,(-)-p-Bromotetramisole Oxalate imidacloprid is rapidly and almost completely absorbed from the gastrointestinal tract. The peak plasma concentration is observed within approximately 2.5 hours and is followed by a rapid disposition phase. However, in our patients the concentrations generally remained elevated for up to 10–15 hours post-ingestion, which might suggest saturation of one or more kinetic pathways in humans at high doses. A possible factor influencing the observed kinetic profile is the administration of atropine which is known to prolong the absorption phase of xenobiotics.
Treatment efficacy is determined by the sensitivity of tumor cells to chemotherapeutic agents
The therapeutic strategy to treat brain metastases depends on the patients’ performance status, systemic tumor activity and the negative impact of older age. Treatment with surgery, radiosurgery and whole brain radiation therapy are the first line therapies for the majority of patients. Although chemotherapy as a single modality has demonstrated limited efficacy, it may improve the result as a concurrent treatment. Overall, there is only limited data on chemotherapeutic protocols from which no firm treatment recommendation can be drawn. Therefore, the chemotherapeutic regimen with highest efficacy to fight the primary tumor in principle is considered also to be the most efficacious for the corresponding brain metastasis. In general, malignant melanoma, renal cell carcinomaand NSCLC show a fairly low chemosensitivity, whereas breast cancer reveal a moderately, SCLC and germ cell cancers a rather high chemosensitivity. The role of TMZ in the treatment of brain metastases is still unclear. Several studies on treating brain metastases with TMZ alone showed low response rates. Preliminary results from randomized trials suggest that combination of TMZ and WBRT is an effective option for patients with brain Epothilone D metastases of non small cell lung cancer. In malignant melanoma, a reduction of mortality from 69% to 41% was observed. For patients with breast cancer and renal cell carcinoma brain metastases, TMZ seems to be less helpful. An obvious possible explanation for variable TMZ efficacy in treating brain metastases is that MGMT promoter methylation has not been investigated systematically in brain metastases. Thus, similarly as for malignant gliomas, where epigenetic silencing of the MGMT gene by promoter methylation has been shown to be of predictive value for profiting from TMZ,Laropiprant TMZ efficacy needs to be correlated to the MGMT promoter methylation status in individual brain metastases. In this study, we show that about one third of brain metastases revealed a methylated MGMT promoter. The methylation rate in the different tumor subgroups ranged between 20% and 46.5%. These results are in line with a previous study on a rather limited number of brain metastases resulting in promoter methylation in,36%. Most studies assessing MGMT promoter methylation status utilize MS-PCR, which is a cost-efficient method requiring only small quantities of DNA. However, DNA derived from FFPEtissue – the routine approach to process tissue for histological assessment and archiving – has been reported to be more often degraded, thus limiting the validity of molecular analyses. On top, bisulfite treatment – a prerequisite for MGMT promoter methylation assays – introduces various additional DNA strand breaks resulting in highly fragmented single stranded DNA. Detection of the MGMT methylation status by 80 cycles of a nested PCR, as recommended for DNA isolated from formalinfixed paraffin-embedded tissue, may easily increase the frequency of sampling error, thus negatively influencing the reliability of results obtained by MS-PCR. This may explain as to why only 61.2% of our samples were evaluable by MS-PCR and why only in 75% of the cases replicate experiments on 20 randomly selected tumor samples yielded reproducible results.
For a fixed number of fungicide applications per crop by using the lowest dose which can provide effective disease control
This result was consistent for a range of fitness costs of resistance and for different degrees of partial resistance. For solo use of a high-risk fungicide, the model output thus suggests that both the part of the effective life spent in the emergence phase and the part of the effective life spent in the selection phase can be maximised. For mixtures of a high-risk and a low-risk fungicide, the median emergence time of resistance to the high-risk fungicide was highest when high dose rates of the low-risk fungicide were combined with the lowest possible dose rate of the high-risk fungicide necessary to provide sufficient disease control of an average epidemic of M. graminicola on winter wheat. Hobbelen et al. determined the number of years that mixtures of a low-risk and a high-risk fungicide can JPH203 provide sufficient disease control in the selection phase for the same host-pathogen system. Similar to the emergence phase, their analysis shows that this number of years is highest when high dose rates of the low-risk fungicide are combined with the lowest possible dose rate of the high-risk fungicide necessary to provide sufficient disease control. It can be concluded that the dose and mixture treatment strategies which are most effective at delaying the evolution of fungicide resistance, do not differ between the emergence phase and the selection phase. The specific model in this paper describes the emergence of resistance to a high-risk fungicide in M. graminicola populations on winter wheat. However, the structure and assumptions underlying the model apply to many foliar fungal pathogens of cereal crops. For example, only parameter values would need to be changed to describe the development of the canopy of cereal crops other than winter wheat. Similarly, the division of the life cycle of fungal pathogens into latent and infectious stages is representative of BTSA1 all fungal pathogens. The division of our model into deterministic and stochastic submodels is to some extent artificial, because stochastic processes will not only influence the dynamics of the resistant strain, but also the dynamics of the host and the sensitive pathogen population. However, such a division is justified when the density of the host and the sensitive pathogen population are so high during most of the growing season that extinction due to stochastic processes is highly unlikely. The advantage of using a deterministic instead of a stochastic model to describe large populations is the much shorter simulation time. There are also a number of limitations to the generality of the model. Firstly, the sensitivity of pathogen strains is assumed to be constant in time. As a result, the model cannot be used to describe a quantitative type of resistance development, characterised by a gradual decrease in sensitivity of the pathogen population due to the accumulation of mutations over time. The best strategy for delaying the emergence of strains with sharply decreased sensitivity due to a single mutation may not be the best strategy for delaying the emergence of strains in which the reduction in sensitivity due to each mutation is relatively small. A second limitation is that the model does not account for the spatial variation in the treatment programs for fungicides.